China-made cancer drug approved for US market

A Chinese pharmaceutical firm has won market approval from the United States Food and Drug Administration for a self-developed lymphoma drug, marking the first entry of an innovative cancer drug from a Chinese company into the US and giving a boost to domestic drugmakers' ambitions to create new treatments for patients around the world.
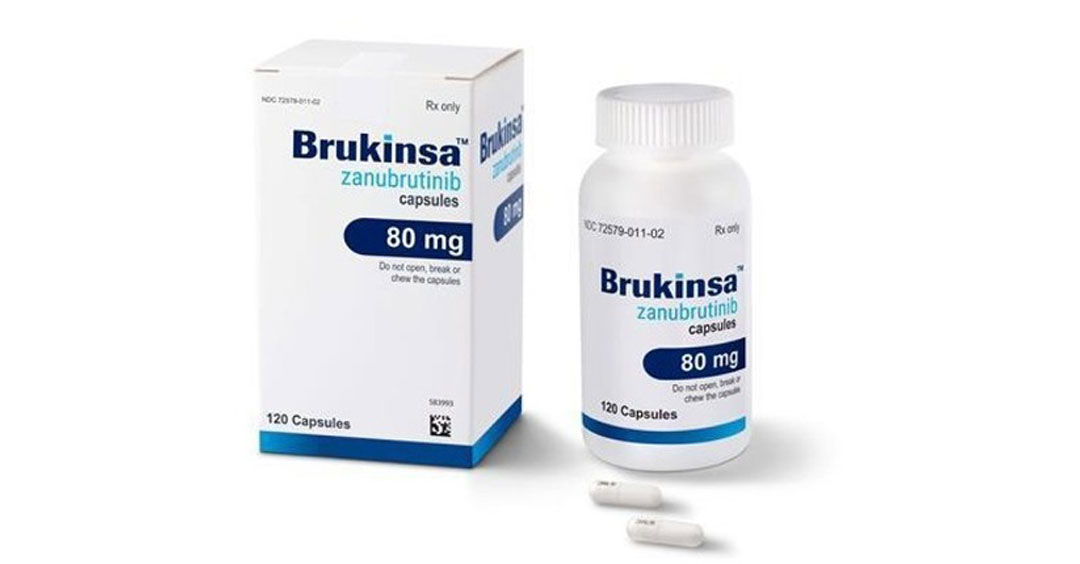
The drug, known as Brukinsa, is a product from Beijing-based BeiGene. It received the FDA's approval on Friday and will probably become available to US patients by the end of the year, Wu Xiaobin, BeiGene president, said at a news conference on Friday.
The application was accepted by the FDA in August, and approval came sooner than originally expected. In January, the drug was granted Breakthrough Therapy designation, an official status that speeds up review processes for drugs that may demonstrate substantial advantages over existing therapies, according to the FDA.
It was also the first time that such a beneficial policy was granted to a Chinese company, according to BeiGene.
"The drug's entry into the US, home of the world's most strictly regulated and advanced pharmaceutical market, will boost other domestic drugmakers' confidence as they eye the global market," he said.
The green light also underlines China's progress in upgrading its pharmaceutical sector from its traditional strategy of making generic drugs to innovation-driven drug development, Wu said.
"Chinese biotech firms are on the rise and are set to benefit patients in developed and developing countries alike," he added.
The company said its application for commercializing the drug in China, which was submitted to the National Medical Products Administration last year, is under review through a domestic fast-track pathway and is expected to be approved in the near future.
The FDA-granted approval is largely based on two sets of data that test its efficacy. The trials were conducted in China over recent years, said Wang Lai, BeiGene's senior vice-president.
"The fact that US market regulators have validated trial results drawn solely from labs in China, the first such validation of its kind, is also of deep significance," Wang said.
With the FDA's blessing, patients with mantle cell lymphoma, a rare and aggressive form of non-Hodgkin's lymphoma, will be able to access this Chinese-developed drug on the condition that they have received at least one prior therapy.
Wang said the company has also stepped up clinical trials using the same drug on other types of blood or lymph tumors.
About two dozen such trial programs are underway around the globe, with several hopeful applicants on track to receive fresh market approval, BeiGene said.
"The new drug tackles a type of malignant tumor that afflicts patients who are in dire need of affordable and effective treatment options and deploys a cell-signaling method, one of the hottest sought-after approaches in drug development around the globe," said Wang Yu, former director-general of the Chinese Center for Disease Control and Prevention.
"The US holds a world-leading drug approval mechanism in terms of its efficiency and rigor, and its approval is very telling of the progress China-based drug firms have achieved over the years," Wang said.
"Chinese companies have begun to play a more active role in new drug innovation, and the news will encourage other domestic drug developers to create urgently needed drugs for patients around the world," Wang said.
While China has made big strides in reforming its healthcare system to speed up drug approvals and cut down delays for new, foreign medicines to reach the market, it has also put greater emphasis on fostering drug innovation and calling for strengthened research and development capability of domestic companies.
Johannes Nippgen, head of R&D Biopharma in China for Merck, a global pharmaceutical giant headquartered in the US, said he had seen more research efforts from Chinese drug developers being devoted to creating novel medications, as opposed to generic versions in recent years.
He added that he did not necessarily see the emergence of Chinese drugmakers as competition. "Ultimately, we are working for the same goal, which is to better treat patients."
Contact the writers at wangxiaoyu@chinadaily.com.cn
- China's Global Governance Initiative receives positive feedback at forum
- China's Xizang sees steady tourism growth in 2025
- First-of-its-kind pearl auction held utilizing Hainan FTP
- Agarwood exhibition steeps Shanghai museum in fragrance
- The Fujian Coast Guard conducts regular law enforcement patrol in the waters near Jinmen
- IP protection for new fields to improve