Prospective vaccine in Phase II trial

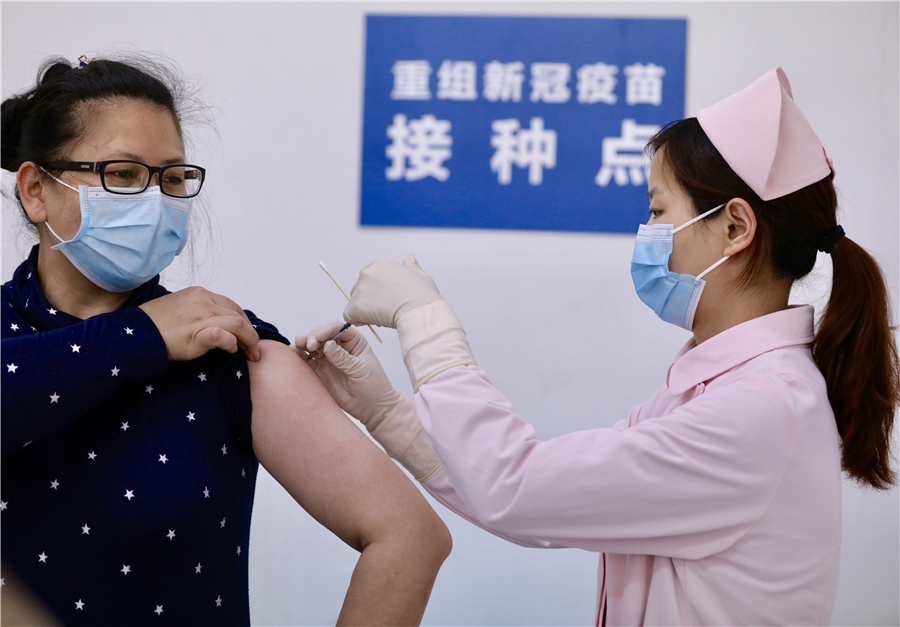
500 symptom-free volunteers to take part in test conducted in Hubei's capital
A recombinant novel coronavirus vaccine, which is China's first vaccine candidate to enter clinical human testing, began recruiting participants for its Phase II clinical trial on Thursday. There is a need for 500 volunteers, five times the number used in the Phase I trial conducted in March.
Participants will be divided into three groups-a moderate dose, a low dose and a control group-according to Cansino Biologics Inc, a Tianjin-based company responsible for the vaccine's development.
It said 250 participants will be involved in the moderate dose test while the rest will be equally distributed into the low dose and control group.
The vaccine candidate is made through recombinant DNA techniques, which typically involve taking a gene from one organism and inserting it into the DNA of another.
The recruitment notification says participants should be aged 18 or above, with neither a previous history of allergies to vaccinations nor a history of COVID-19 infection.
The trial will be conducted in Wuhan, the hardest-hit region in China, so participants are also required to have a "green code"-an indication of being symptom free according to a health code app, which includes basic health information and travel history-which was developed due to the pandemic.
The vaccine candidate, known as Ad5-nCoV, was jointly developed by Cansino and the Institute of Biotechnology of the Academy of Military Medical Sciences.
The Phase I trial, which included 108 test subjects, took place at Tongji Hospital in Wuhan, Hubei province, last month. According to the Chinese Clinical Trial Registry, the single-center, open and dose-escalation Phase I trial tested the safety of and tolerance for the vaccine candidate in healthy adults.
Adverse reactions seen in the Phase I trial included fever and pain at the inoculation site and the joints. The group that received a high dose reported more fevers, but the symptom usually disappeared within 24 hours.
Unlike the Phase I trial that required participants to undergo 14-day quarantine restrictions in a designated place after being vaccinated, the Phase II clinical trial allows participants to conduct self-quarantine at home.
In the six months after vaccination, investigators will continue to monitor all participants and visit each one four times.
The COVID-19 vaccine trial in China follows the first trial launched in the United States at Kaiser Permanente Washington Health Research Institute in Seattle, Washington, which evaluated the US drug company Moderna's mRNA-1273 vaccine in mid-March. Moderna's trial used a gene-based method that uses messenger RNA to trigger an immune response in the target individual. Another candidate, the INO-4800 DNA vaccine by Inovio Pharmaceuticals in the US, also entered Phase I clinical human testing this month.
The Ad5-nCoV in China is developed with Cansino's adenoviral vector-based vaccine, a platform which was key to enabling the company to develop its Ebola virus vaccine, Ad5-EBOV, in only three years.
Zheng Zhongwei, an official at the National Health Commission, said at a recent news conference that there are currently eight institutions in China working on developing vaccines along five technical routes.
He said all vaccine research is carried out according to appropriate laws, standards and norms. "Our goal is to make sure the vaccine against the novel coronavirus will be put into use as soon as possible, based on the premise of ensuring its safety and effectiveness," Zheng said.
Zou Shuo contributed to this story.