Vaccine makers may face harsh penalties for legal violations

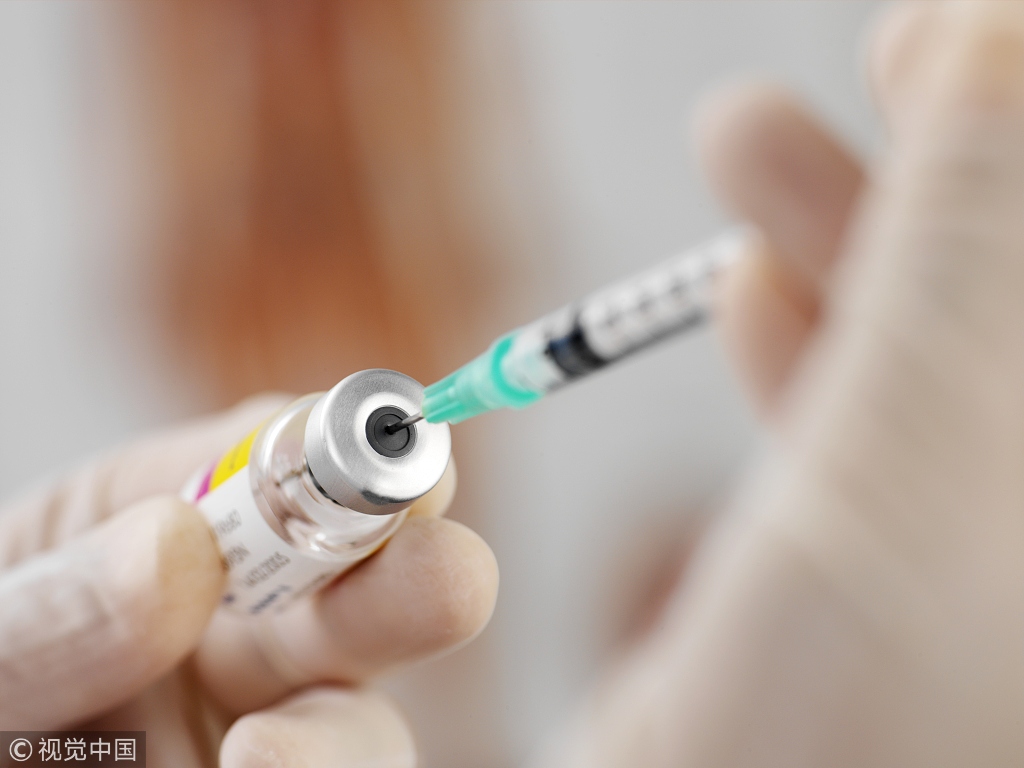
Vaccine producers could be given harsh penalties for a range of violations, with substantial fines possible, under a new draft law from China's top market regulator after a major quality control scandal earlier this year.
Serious violators could have profits confiscated, certificates revoked, production suspended and face fines of up to 10 times the value of the products in question.
Violations under scrutiny include falsifying manufacturing records and failing to recall products sold after quality issues or other potential safety risks were discovered, according to the draft.
Senior executives and other employees at key posts within offending companies will have their salaries recalled during periods of infraction, and may be required to pay an additional fine of between 50 and 100 percent of their pay. They may be banned from engaging in pharmaceutical activities for life, according to the draft.
The draft was released by the State Administration for Market Regulation on its website and is soliciting public opinion and feedback until Nov 25.
Almost all existing laws were drafted by government departments before they were submitted to legislators for consideration.
Producers of substandard drugs could have their illegal gains confiscated and pay fines of up to three times the value of the products involved, according to the existing Drug Management Law, which covers vaccines and is now undergoing revision.
The draft, once made into law, will prioritize the Drug Management Law and other related laws in the management of vaccines, according to the administration.
Vaccine producers could have their illegal gains confiscated and production suspended, and they would face fines of up to 1 million yuan ($143,000) for violations such as refusing to take effective measures to recall problematical products sold on the market, the draft said.
Vaccine producers must purchase insurance in case they are faced with compensation for substandard products, it said.
"Vaccines relate to people's life and health, public health and national security," the administration said in a statement.
The draft was made in response to public concern over improving supervision of vaccines, and is meant to eliminate loopholes exposed by the case involving Changchun Changsheng Bio-tech Co, it said.
The rabies vaccine producer, based in Changchun, Jilin province, was found to have falsified production records during an unannounced inspection in July by China's top drug authority.
An investigation team announced in August the company had committed other serious violations such as using expired raw materials.
Rabies is almost always fatal if untreated. No injuries or deaths caused by the substandard rabies vaccines produced by the company have been publicly reported so far.
Tao Lina, a Shanghai-based public health expert, said the punitive measures stipulated in the draft will help deter vaccine producers and sellers from violating laws. But how to effectively uncover such violations remains a problem.
"A system that financially rewards whistlerblowers should be established to encourage insiders to report law violations," he said.
Wang Yuedan, a professor of immunology at Peking University, said requiring vaccine producers to buy insurance will ensure compensation for those receiving vaccinations if serious side effects occur, and encourage vaccinations among the public.
"Although many regulations stipulated in the draft have in practice been carried out, they will be better implemented once they become law," he said.
- Xi receives credentials of new ambassadors to China
- South Korean visitor praises Ningxia's spicy street food
- Galactic Energy completes sixth sea-based launch of Ceres 1 rocket
- China's giant radio telescope observations unravel origin of cosmic enigmatic flashes
- Xi meets Canadian prime minister
- Xinjiang official, famed as a 'rural influencer', dies after fall from horse